Abstract
Background: Improved treatments for Hodgkin and non-Hodgkin lymphoma has resulted in a growing population of long-term survivors, many of whom are cured. A focus of long-term care of these patients (pts) has in turn moved toward optimizing survivorship, including identification and management of second primary malignancies (SPM). In this prospective cohort study initiated in the modern treatment era, we describe the incidence and factors associated with risk of SPM.
Methods: Pts were identified from the Molecular Epidemiology Resource (MER) cohort of the University of Iowa/Mayo Clinic Lymphoma SPORE, which offers enrollment to all consecutive patients with newly diagnosed lymphoma aged 18+ years. Clinical and treatment data are abstracted from medical records using a standard protocol. Pts are prospectively contacted regularly to assess disease status and development of SPM. All reported SPM through 2021 were validated against medical records. For this analysis, SPM were defined as any cancer other than lymphoma diagnosed >90 days from lymphoma diagnosis. Cumulative incidence of SPM and 95% confidence intervals (CI) were calculated with death as a competing risk, and associations with patient and treatment characteristics were estimated with Cox models. To evaluate the incidence of SPM in the MER against expected population rates, we calculated the standardized incidence ratio (SIR) using the Iowa component of the Surveillance, Epidemiology and End Results (SEER) database, accounting for age, sex, calendar year and person-time.
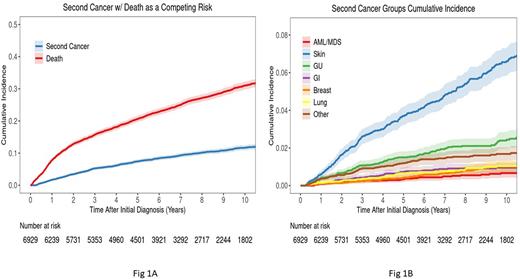
Results: Of 6929 pts enrolled into the MER from 9/2002 - 6/2015, 59% were male; median age at enrollment was 61 yrs (IQR: 51-70). 763 pts developed SPM through 2021, including 37 acute myeloid leukemia and myelodysplastic syndrome (AML/MDS), 341 non-melanoma skin, 133 genitourinary (GU), 57 gastrointestinal (GI), 49 breast, and 52 lung cancers. The 10-year cumulative incidence of SPM was 12% (CI: 11-13), and 31% (CI: 30-32) for the competing risk of death (Fig 1A). Skin cancers had highest 10-year cumulative incidence of 6.6% (CI: 5.9 -7.4), followed by GU 2.4% (CI: 2.0-2.9), breast 1.0% (CI: 0.8-1.4), lung 1.0% (CI: 0.8-1.3), GI 0.9% (CI: 0.7-1.3), and AML/MDS 0.7% (CI: 0.5-1.0)(Fig 1B). The MER cohort had a lower than expected rate of any SPM (excluding non-melanoma skin cancer, which SEER does not collect) compared to the general Iowa population (SIR=0.61, CI: 0.5-0.7), as well as lower than expected rates of GU (SIR=0.60, CI: 0.5-0.7), GI (SIR=0.45, CI: 0.3-0.6), breast (SIR=0.69, CI: 0.5-0.9), and lung (SIR=0.47, CI: 0.4-0.6) cancer. In contrast, the MER showed a 5-fold higher than expected rate of AML/MDS (SIR=5.2, CI: 3.6-7.1). Risk of SPM increased with age: compared to pts age 18-40 years, those 41-60 yrs had a 3-fold (CI: 1.9-4.5) and >60 yrs had a 6.3-fold (CI: 4.2-9.6) elevated risk of SPM. Females were at lower risk of SPM (HR=0.7, CI: 0.6-0.9). After adjustment for age and sex, there was no association with lymphoma subtype. For initial treatment class (adjusted for age, sex and subtype), risk of a SPM was associated with use of a systemic chemotherapy (HR=1.3, CI: 1.1-1.6); was inversely associated with initial observation (HR=0.8, CI: 0.6-0.9); and was not associated with use of local radiation (HR=0.9, CI: 0.7-1.2), an anthracycline (HR=1.0, CI: 0.8-1.3), alkylator (HR=1.2, CI: 1.0-1.4) or R-monotherapy (HR=1.0, CI: 0.7-1.3). In joint modeling of initial treatment adjusted for age, sex and subtype, compared to pts not receiving systemic chemotherapy, pts with systemic therapy including an alkylator but no anthracycline (HR=1.4, CI: 1.1-1.7) or systemic therapy without an alkylator or anthracycline (HR=1.3, CI:1.1-1.7) had increased risks of SPM, while those receiving both an alkylator/anthracycline were not at significantly elevated risk (HR=1.2, CI: 0.9-1.5).
Conclusions: In the rituximab era, the cumulative incidence of any SPM increased over time and at 10 years after diagnosis was 12%, with about half of these due to non-melanoma skin cancers. While the cumulative incidence of AML/MDS at 10 years was low (0.7%), this represented a 5-fold excess risk over expected population rates, consistent with prior eras. In contrast, solid tumors were below expected population rates. Older age, male sex and systemic chemotherapy were associated with higher risk of SPM. Prevention and management of SPM continues to remain an important need for lymphoma survivors.
Disclosures
Wang:Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; InnoCare: Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo@Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Genentech: Research Funding; MorphoSys: Research Funding; Genmab: Research Funding; Eli Lilly and Company: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees. Farooq:MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Honoraria; Caribou pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Checkmate Pharma: Research Funding. Nowakowski:Bantam Pharmaceutical: Consultancy; Blueprint Medicines Corporation: Consultancy; Celgene Corporation/Bristol Myers Squibb: Consultancy, Research Funding; Curis, Inc.: Consultancy; Daiichi Sankyo Inc: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Genentech, Inc: Consultancy, Research Funding; Incyte: Consultancy; Karyopharm: Consultancy; Kite Pharma Inc.: Consultancy; Kymera Therapeutics: Consultancy; MorphoSys US Inc: Consultancy; NanoString: Research Funding; Ryvu Therapeutics: Consultancy; Selvita: Consultancy; TG Therapeutics: Consultancy; Zai Lab: Consultancy. Maurer:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Roche/Genentech: Research Funding. Kay:Rigel: Other: Data Safety Monitoring Committee; Morpho-sys: Other: Data Safety Monitoring Committee; Dren Bio: Other: Data Safety Monitoring Committee; BMS: Other: Data Safety Monitoring Committee, Research Funding; Targeted Oncology: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Committee; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Cytomx Therapy: Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Committee; Behring: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Committee; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tolero Pharmaceuticals: Research Funding; TG Therapeutics: Research Funding; Sunesis: Research Funding; MEI Pharma: Research Funding; Genentech: Research Funding; Celgene: Other: Data Safety Monitoring Committee, Research Funding. Link:Jannsen: Research Funding; Bristol-Myers Squibb: Research Funding; MEI: Consultancy; Novartis: Research Funding; Genentech / Roche: Consultancy, Research Funding. Cerhan:BMS/Celgene: Research Funding; Genentech: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; NanoString: Research Funding; Protagonist: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal